
REBEL Cast
Salim R. Rezaie, MD
Rational Evidence Based Evaluation of Literature
- 5 minutes 7 secondsREBEL Core Cast 121.0 – Acute Sinusitis

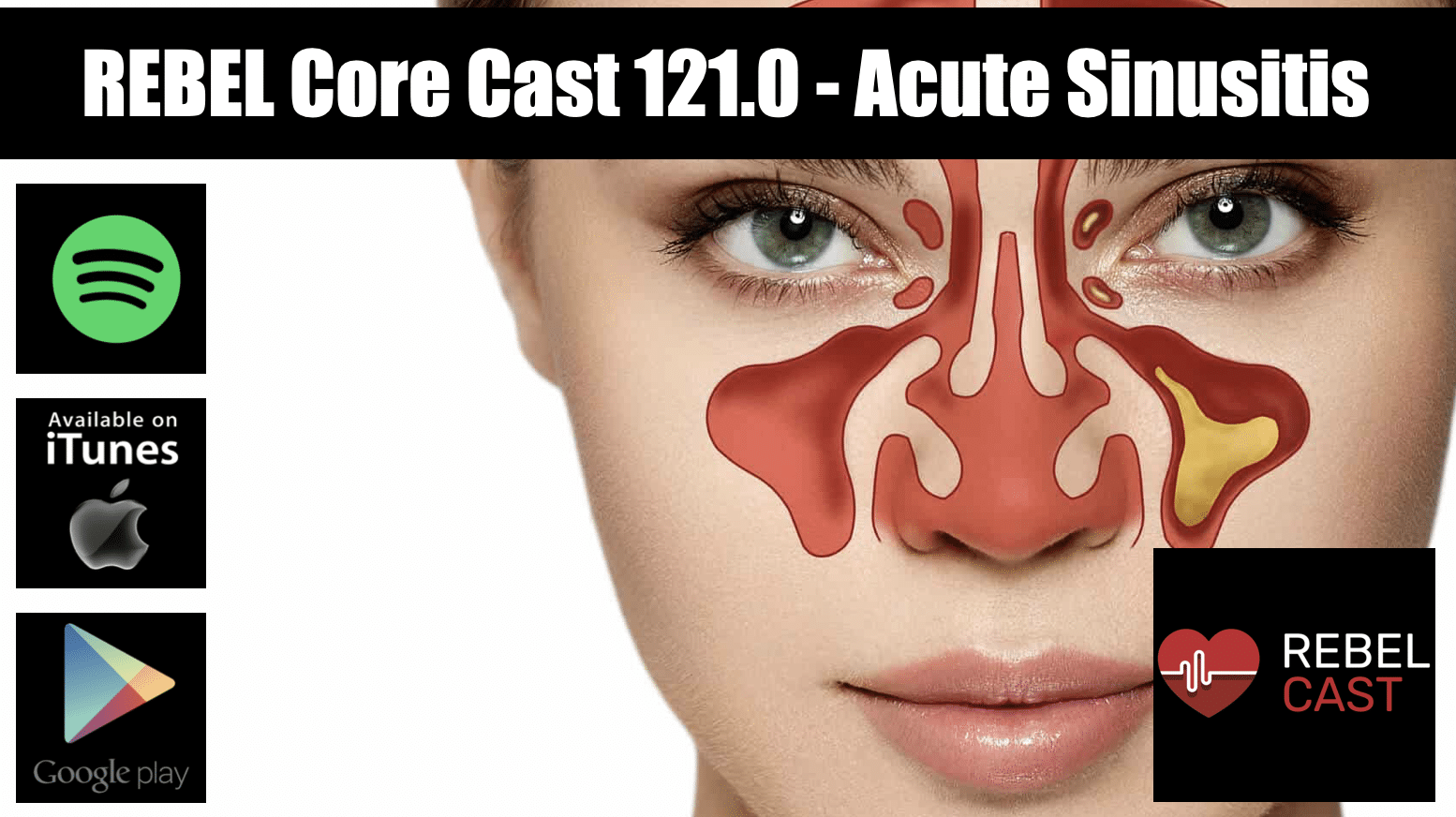 Take Home Points
Take Home Points- Acute rhinosinusitis is a clinical diagnosis
- The vast majority of acute rhinosinusitis cases are viral in nature and do not require antibiotics
- Consider the use of antibiotics in select groups with severe disease or worsening symptoms after initial improvement.
REBEL Core Cast 121.0 – Acute Sinusitis
Click here for Direct Download of the Podcast.
Definition:
- Acute rhinosinusitis (ARS) – Symptoms for less than four weeks
- Subacute rhinosinusitis – Symptoms for 4 to 12 weeks
- Chronic rhinosinusitis – Symptoms persisting greater than 12 weeks
- Recurrent acute rhinosinusitis – Four or more episodes of ARS per year, with interim symptom resolution
Epidemiology: (Anon 2004)
- 20 million cases of sinusitis annually in the US, costing $3.5 billion/year
- Source of 1 in 5 antibiotic prescriptions for adults
Presentation:
- Sinusitis is most commonly diagnosed by clinical symptoms
- Common symptoms
- Purulent nasal discharge
- Nasal congestion
- Facial pain or pressure, especially over a sinus or unilaterally
- Anosmia
- Hyposmia
- Fever
- Cough
- Fatigue
- Maxillary pain
- Ear pressure or fullness.
Classification of Sinusitis:
●Acute viral rhinosinusitis (AVRS)
- ARS with viral etiology (i.e. rhinovirus, influenza, and parainfluenza)
- Most common form of ARS
●Uncomplicated acute bacterial rhinosinusitis (ABRS)
- ARS with a bacterial etiology without clinical evidence of extension outside the paranasal sinuses and nasal cavity
- Bacterial superinfection: 0.5-2% of all ARS
●Complicated acute bacterial rhinosinusitis
- ARS with bacterial etiology with clinical evidence of extension outside the paranasal sinuses and nasal cavity
Sinusitis: Viral vs. Bacterial:
- Color change in sputum does not determine whether infection is viral or bacterial
- Viral infections
- Tend to begin resolution by 7-10 days
- Rarely have associated fevers
- If fever present, usually only in the first 48 hours.
- Guidelines for diagnosing ABRS are
- Presence of URI/cold symptoms that
-
-
-
- Don’t improve after 10 days
- Worsen after 5-7 days of improvement
- Severe symptoms including high fever, purulent discharge or facial pain for 3-4 days
-
-
The Data Behind Antibiotic Use
-
- Clinically diagnosed acute sinusitis
-
- Multiple studies show the same cure rate at 7 days, but improved cure rate at 7-14 days for those who use antibiotics (Lemiengre 2012, Berg 1986, Gwaltney 1996)
- Overall Treatment Effect NNT = 18
- Overall Harm NNH = 8 (mostly GI side effects)
- Radiographically-diagnosed acute sinusitis (Ahovuo-Saloranta 2008)
- Endpoint: clinical cure at 7-15 days
- NNT = 15
- NNH = 8
-
- Clinically diagnosed acute sinusitis
IDSA Recommendations for Antibiotic Treatment (Chow 2012)
- Patients that should be treated
- Persistent symptoms w/o improvement (> 10 days)
- Severe symptoms (> 3-4 days)
- Worsening (“double-sickening”) (> 3-4 days)
- Antimicrobials
- 1st Line
- Amoxicillin 875 mg PO BID X 5-7 days
- Doxycycline 100 mg PO BID X 5-7 days
- 2nd Line
- Amoxicillin/Calvulanate 875/125 mg PO BID X 5-7 days
- Levofloxacin 500 mg PO Q24 X 5 days
- 1st Line
Bottom Line: Given the risk for adverse events associated with antibiotic use, the growing specter of resistance and the lack of significant differences in outcomes with antibiotic use, it is better to avoid antibiotics in most patients with ARS. Antibiotics should be considered in those with severe disease and in immunocompromised patients
Take Home Points
- Acute rhinosinusitis is a clinical diagnosis
- The vast majority of acute rhinosinusitis cases are viral in nature and do not require antibiotics
- Consider the use of antibiotics in select groups with severe disease or worsening symptoms after initial improvement.
References
- Anon JB et al. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg 2004; 130(Suppl 1): 1-45. PMID: 14726904
- Lemiengre MB et al. Antibiotics for Clinically Diagnosed Acute Rhinosinusitis in Adults. Cochrane Database Syst Rev 2012. PMID: 23076918
- Berg O et al. Occurence of asymptomatic sinusitis in common cold and other acute ENT-infections. Rhinology 1986; 24(3): 223-5. PMID: 3775189
- Gwaltney JM. Acute community-aquired sinusitis. Clin Infect Dis 1996; 23(6): 1209-23. PMID: 8953061
- Ahovuo-Saloranta A et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev 2008. PMID: 18425861
- Chow AW et al. IDSA Clinical practice guideline for acute bacterial rhino sinusitis in children and adults. Clin Infect Dis 2012; 54(8): e72-e112. PMID: 22438350
Read More
- The NNT.com: Antibiotics for Clinically Diagnosed Acute Sinusitis in Adults
- The NNT.com: Antibiotics for Radiologically-Diagnosed Acute Maxillary Sinusitis
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter/X: @srrezaie)
The post REBEL Core Cast 121.0 – Acute Sinusitis appeared first on REBEL EM - Emergency Medicine Blog.
1 May 2024, 3:00 pm - 39 minutesREBEL EM Book Club – MicroSkills


Podcast Direct Download: Link
Release Date: April 16th, 2024
Show Notes
Dr. Glaucomflecken: Power of Ultrasound with Emergency Medicine Dr. Resa Lewiss
Adaira I Landry MD
Resa E Lewiss MD is a Professor of Emergency Medicine at the University of Alabama at Birmingham. A TEDMED speaker and TimesUp Healthcare founder, she’s an internationally renowned point-of-care ultrasound educator and champion for diverse, equitable, and inclusive workplaces. She attended college at Brown, medical school at Penn, Emergency Medicine residency at Harvard, and fellowship at Mount Sinai St. Luke’s Roosevelt. She led point-of-care ultrasound sections at St. Luke’s Roosevelt, the University of Colorado, and Thomas Jefferson. A physician healthcare design consultant for Perkins&Will, her design focus has been ultrasound hardware and workflows. She’s helped to redesign the built environment of a Harvard ICU and an infectious diseases unit in Malawi. As host and founder of the Visible Voices Podcast, she’s interviewed dozens of subject matter experts in healthcare, equity, and current trends. Her writings are published in the popular press and scientific journals, such as Harvard Business Review, Slate, Nature, and Fast Company. Her new book, MicroSkills : Small Actions, Big Impact is forthcoming from HarperCollins in 2024.
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter/X: @srrezaie)
The post REBEL EM Book Club – MicroSkills appeared first on REBEL EM - Emergency Medicine Blog.
9 April 2024, 3:30 pm - 34 minutes 50 secondsREBEL Cast – EMTALA + Reproductive Health Rights

REBEL Cast – EMTALA + Reproductive Health
Click here for Direct Download of the Podcast.
Dr. Dara Kass is a practicing emergency medicine physician who was most recently as the Regional Director of Region 2 for the US Department of Health and Human Services.
She currently works with organizations and institutions to advance and implement policies that affect the care of individuals in this new healthcare landscape, most specially related to all forms of reproductive health care from contraception and pregnancy termination to addressing the maternal mortality crisis in the United States.. Doctor Kass’s impact is broad as a tireless advocate, spearheading initiatives, and campaigns formatting improvements on issues such as gender equity, reproductive healthcare, and organ donation. Doctor Kass lives in Scarsdale NY with her husband and three children.
Dr. Monica Saxena is a practicing emergency physician and assistant professor at Stanford University School of Medicine.
Her research focuses on reproductive justice and women’s health in the emergency department setting. Dr. Saxena is the 2022 recipient of the Rising Star Faculty Award from the American College of Emergency Physicians. She holds a law degree from the University of Michigan and a medical degree from Wayne State University School of Medicine.
MedPage Today: The Ethos of Emergency Medicine Hangs in the Balance
Resources:
Reproductiverights.gov
Submit your complaint to the State Survey Agency in the state where the hospital is located.
The post REBEL Cast – EMTALA + Reproductive Health Rights appeared first on REBEL EM - Emergency Medicine Blog.
3 April 2024, 3:00 pm - 17 minutesREBEL Cast Ep125: 1st 48 Hours of PE Management – How Good Is Unfractionated Heparin?

 Background: The mainstay of treatment for symptomatic pulmonary embolism (PE) is anticoagulation (AC). Patients with higher-risk PE may require advanced interventions such as thrombolytic therapy, surgical thrombectomy, or even extracorporeal membrane oxygenation (ECMO). Because of its short half-life and availability of a reversal agent, unfractionated heparin (UFH) is commonly used when percutaneous or surgical interventions are being considered.
Background: The mainstay of treatment for symptomatic pulmonary embolism (PE) is anticoagulation (AC). Patients with higher-risk PE may require advanced interventions such as thrombolytic therapy, surgical thrombectomy, or even extracorporeal membrane oxygenation (ECMO). Because of its short half-life and availability of a reversal agent, unfractionated heparin (UFH) is commonly used when percutaneous or surgical interventions are being considered.The standard weight based dosing of UFH is 80U/kg bolus followed by an infusion started at 18U/kg/hr, titrated to a target activated partial thromboplastin time (aPTT) of 1.5 to 2.5x the control range or an anti-Xa level of 0.3 to 0.7u/mL. The efficacy of UFH in reaching and maintaining appropriate anticoagulation is poorly understood.
REBEL Cast 125: 1st 48 Hours of PE Management – How Good is Unfractionated Heparin?
Click here for Direct Download of the Podcast
Paper: Prucnal CK et al. Analysis of Partial Thromboplastin Times in Patients With Pulmonary Embolism During the First 48 Hours of Anticoagulation With Unfractionated Heparin. Acad Emerg Med 2020. PMID: 31625654
Clinical Question: How effective is UFH in obtaining appropriate anticoagulation during the first 48 hours of administration to patients with acute PE?
What They Did:
- Retrospective analysis of a PE response team (PERT) data base
- Single, large, urban academic teaching hospital (Massachusetts General Hospital)
- October 2012 to April 2017
- 2 Standard Dosing Regimens Evaluated
- Bolus + Drip (Low Risk of Bleeding): 80U/kg + Continuous titrated infusion starting at 18U/kg/hr
- Drip Only (Higher Risk of Bleeding): Continuous titrated infusion starting at 18U/hr
- Subsequent aPTT (seconds) and Rate Changes
- <40: +3U/kg/hr
- 40 – 49.9: +2U/kg/hr
- 50 – 49.9 +1U/kg/hr
- 60 to 80: No change
- 1 to 100: -2U/kg/hr
- >100: Hold for 60minutes then -3U/kg/hr
Outcomes:
- Proportion of patients with a therapeutic aPTT value during each 6 hour time period
- A therapeutic aPTT was defined as a value of 60 to 80seconds
- A therapeutic Anti-Xa level was defined as a value of 0.3 to 0.7u/mL
Inclusion:
- Adult patients
- Acute PE
- PERT team consulted
- Received anticoagulation with UFH according to guideline standard dosing
Exclusion:
- Patients treated with nonstandard dosing regimens
Results:
- 505 patients met inclusion criteria
- PE Severity
- Saddle: 17.4%
- Rt Heart Strain on CT: 46.9%
- Hemodynamic Collapse: 7.9%
- Massive PE: 28.3%
- Right Heart Strain on Echo: 21.4%
- Elevated Troponin Level: 55.3%
- Interventions Received:
- Systemic thrombolysis: 4.0%
- Catheter-Based Intervention: 5.9%
- Surgical Thrombectomy: 2.0%
- ECMO: 1.4%
- IVC Filter: 5.0%
- 30d Outcome:
- Mortality: 5.7%
- Re-Thrombosis: 3.9%
- Bleeding: 3.0%
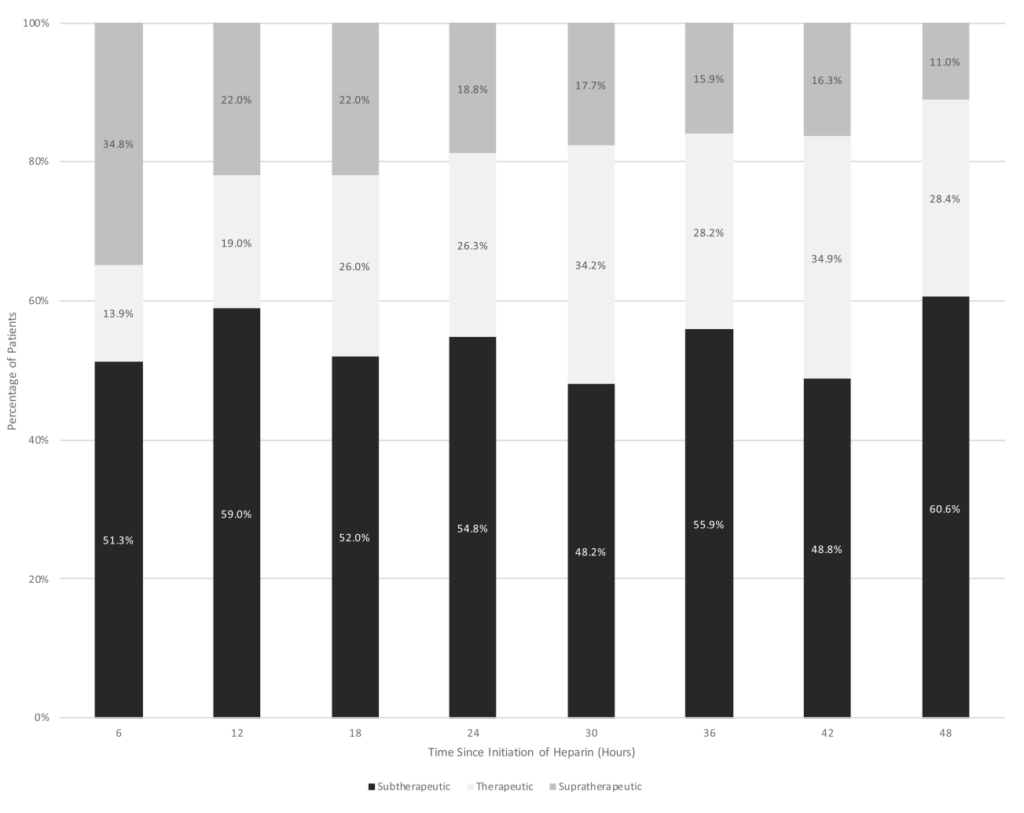
- Therapeutic aPTT in patients receiving bolus and infusion of UFH
- At 6hrs: 13.9% (10.2 to 18.5%)
- At 12hrs: 19% (14.2 to 25.0%)
- At 24hrs: 26.3% (25.3 to 33.1%)
- At 36hrs: 28.3% (22.0 to 35.4%)
- At 48hrs: 28.4% (20.8 to 37.5%)
- PE Severity
- Therapeutic aPTT in patients receiving infusion only of UFH
- At 6hrs: 14.5% (9.5 to 21.5%)
- At 12hrs: 23.3% (15.2 to 32.3%)
- At 24hrs: 41.4% (31.6 to 51.9%)
- At 36hrs: 37.0% (26.8 to 48.5%)
- At 48hrs: 42.1% (30.2 to 55.0%)
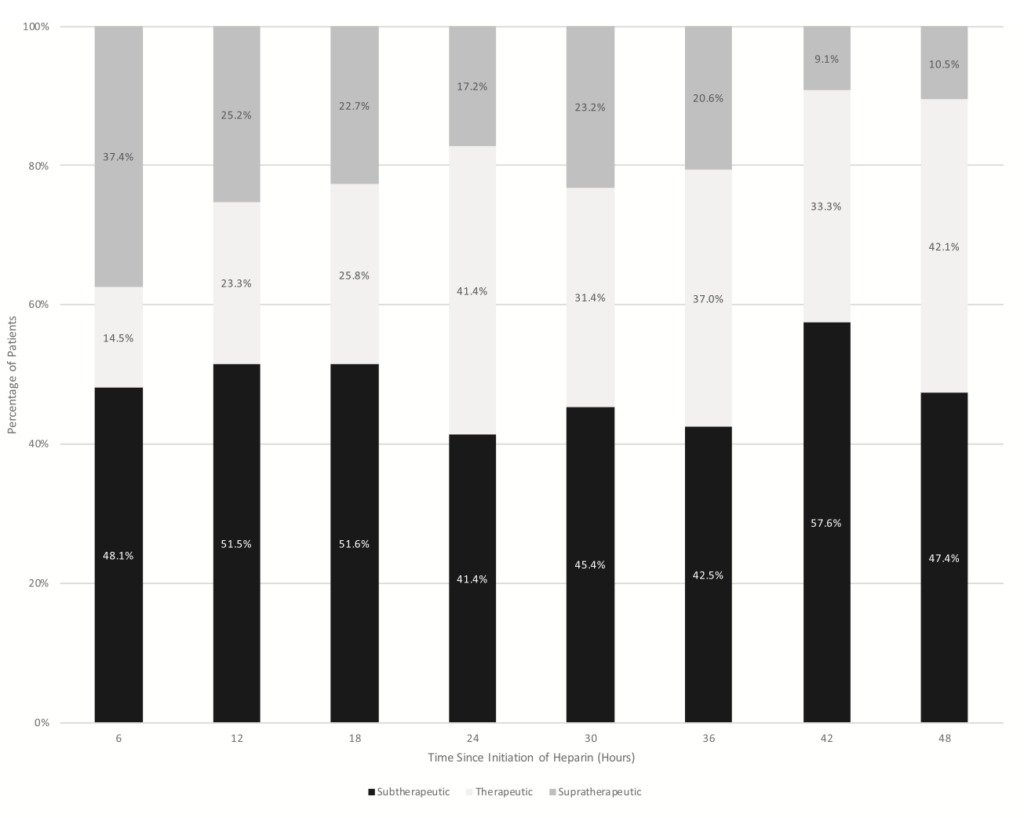
- No patients had all therapeutic aPTT values
Strengths:
- Asks a clinically important question
- Study was stratified for patients being treated with bolus plus titrated infusion or titrated infusion only
- Used UFH standard dosing strategies used by most physicians
- Performed a sensitivity analysis that excluded all patients treated with systemic or catheter-directed thrombolysis, surgical embolectomy, or ECMO
Limitations:
- Initiation of AC was defined as the order start time for UFH in the electronic medical record. There could have been delays in actually starting the UFH
- No real details on chart extraction methodology (i.e. how they handled incomplete or conflicting data)
- Only one abstractor where there is no assessment of the abstractors performance or comparison to another abstractor
- Unclear why one strategy was chosen over another
- Selection bias: Only patients consulted by PERT
- For patients with more than one aPTT value in a given 6 hour time period, the first therapeutic value was selected for analysis. If there was no therapeutic value, the first aPTT value reported was used
- Single center study meaning local factors may limit generalizability to other institutions
- Time outside therapeutic aPTT is not all equal. An aPTT that is slightly above or below the reference range would be considered outside the range (i.e. values close to the therapeutic cutoff were considered equivalent to those far from the cut-off range)
- This study does not determine whether time spent in the therapeutic range affects morbidity and mortality (Very low mortality rate = 6%)
Discussion:
- Only a minority of patients in whom the PERT team was consulted treated with UFH using standard dosing had a therapeutic aPTT during the first 48 hours of anticoagulation with the majority of patients being subtherapeutic
- The proportion of patients in therapeutic range was lowest at 6 hours (14%) and highest at 42 hours (35%)
- Approximately 40% of patients failed to reach the therapeutic range in the first 48 hours of AC
- It wasn’t until 36 hours after the initiation of UFH that >50% of patients had at least one therapeutic aPTT
- There were no patients who had all aPTT values within the therapeutic range during the first 48hours of UFH therapy
- Something to Think About: Thrombus burden can be substantial and heparin resistance may be present, so standard dosing may be inadequate in these patients
- My Opinion: LMWHs offer several advantages over unfractionated heparin including a longer half-life, increased bioavailability, and a more predictable dose response. In addition, LMWHs are dosed by weight, administered subcutaneously, and usually do not require dose adjustments or laboratory monitoring. Whereas unfractionated heparin is largely hepatically cleared, LMWHs are renally cleared
- In 2017 [2], there was a Cochrane review that compared LMWH to UFH for the initial treatment of VTE. This included 29 RCTs with over 10,000 patients. The authors concluded that with moderate quality of evidence fixed dose LMWH reduced the incidence of recurrent thrombotic complications and occurrence of major hemorrhage during initial treatment with no difference in overall mortality compared to UFH
- The 2019 European Society of Cardiology [3] also states: “LMWH and fondaparinux are preferred over UFH for initial anticoagulation in PE, as they carry a lower risk of inducing major bleeding and heparin-induced thrombocytopenia. Neither LMWH nor fondaparinux need routine monitoring of anti-Xa levels. Use of UFH is nowadays largely restricted to patients with overt hemodynamic instability or imminent hemodynamic decompensation in whom primary reperfusion treatment will be necessary. UFH is also recommended for patients with serious renal impairment [creatinine clearance ≤30mL/min] or severe obesity. If LMWH is prescribed in patients with CrCl 15 – 30mL/min, an adapted dosing scheme should be used.”
Author Conclusion: “The majority of patients with acute PE spend most of their first 48 hours outside of the therapeutic range of AC when treated with guideline standard dosing of UFH. Over half of the patients fail to achieve any therapeutic PTT level within 24 hours of UFH initiation, and no patient had all therapeutic aPTTs. Future research should focus on identifying factors associated with achieving therapeutic AC with UFH.”
Clinical Take Home Point: In this single center study of PERT team consulted PE patients standard dosing of UFH left most patients with a subtherapeutic aPTT level in the first 48 hours of treatment. Either we need to question the dosing regimen we use for UFH or we should consider switching to LMWH in the initial treatment of PE patients.
References:
- Prucnal CK et al. Analysis of Partial Thromboplastin Times in Patients With Pulmonary Embolism During the First 48 Hours of Anticoagulation With Unfractionated Heparin. Acad Emerg Med 2020. PMID: 31625654
- Robertson L et al. Fixed Dose Subcutaneous Low Molecular Weight Heparins Versus Adjusted Dose Unfractionated Heparin for the Initial Treatment of Venous Thromboembolism. Cochrane Database Syst Rev 2017. PMID: 28182249
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter/X: @EMSwami)
The post REBEL Cast Ep125: 1st 48 Hours of PE Management – How Good Is Unfractionated Heparin? appeared first on REBEL EM - Emergency Medicine Blog.
1 April 2024, 12:00 pm - 12 minutes 21 secondsREBEL Cast Ep124: Nitrates in Right Sided MIs?

 Background: Nitrates can help improve symptoms and ischemia in the setting of acute myocardial infarction. Current teaching holds that nitrates should be avoided in patients with potential right ventricular myocardial infarction (RVMI), due to the risk of decreasing preload and precipitating hypotension. This belief is based on a single 1989 study of 40 patients with RVMI and endorsed by both the AHA and ESC guidelines [2].
Background: Nitrates can help improve symptoms and ischemia in the setting of acute myocardial infarction. Current teaching holds that nitrates should be avoided in patients with potential right ventricular myocardial infarction (RVMI), due to the risk of decreasing preload and precipitating hypotension. This belief is based on a single 1989 study of 40 patients with RVMI and endorsed by both the AHA and ESC guidelines [2].In that 1989 study, of the 40 patients with RV infarction 20 had a decrease in blood pressure of ≥30mmHg and associated symptoms after the administration of nitrates (SL, Oral, Transdermal, or IV) and 20 did not. The conclusion of this paper was that inferior AMI with RV involvement has a strong association with hypotensive response to nitrates. The major issue is the study is limited by the fact that we are given no information on dosage and multiple routes of administration making clinical application difficult. Better data is needed to guide management.
REBEL Cast 124: Nitrates in Right Sided MIs?
Click here for Direct Download of the Podcast
Paper: Wilkinson-Stokes M et al. Adverse Events From Nitrate Administration During Right Ventricular Myocardial Infarction: A Systematic Review and Meta-Analysis. Emerg Med J 2023. PMID: 36180168
Clinical Question: Is giving nitrates to patients with right ventricular myocardial infarction (RVMI) associated with increased adverse events compared with nitrate administration to patients with myocardial infarctions in other regions of the heart?
What They Did:
- A systematic review and meta-analysis
- Exposure of interest: administration of nitrates, via any route and dose
Outcomes:
- Primary: All forms of adverse events reported in the included studies (Hypotension, Bradycardia, AMS, Syncope, Cardiac Arrest)
Inclusion:
- Patients diagnosed with AMI with a subset of the sample with RV myocardial infarction
- Experimental and analytical observational study designs
- Studies only published in English
Exclusion:
- Patients with coronary vasospasm
Results:
- 5 studies included in the analysis
- Only 2 observational studies using SL NTG 400mcg were used for the meta-analysis
- Adverse Events (Hypotension, Bradycardia, AMS, Syncope, Cardiac Arrest)
- RVMI: 18/105 (17.1%)
- Other MIs: 83/945 (8.8%)
- RR 1.31 (95% CI 0.81 to 2.12)
- No patients had cardiac arrest or death
- Adverse Events (Hypotension, Bradycardia, AMS, Syncope, Cardiac Arrest)
Strengths:
- Asks a clinically important question
- Searched 6 different databases for relevant papers
- All included studies underwent quality assessment using standard appraisal tools
- If data was missing corresponding authors were contacted for the missing information
Limitations:
- None of the included studies were RCTs
- All studies samples were of combined inferior and RVMI making it difficult to determine the safety of nitrates during isolated RVMI
- One of the included studies was only an abstract
- Studies defined hypotension in different ways which could alter frequency of adverse events
Discussion:
- Overall this review provides low certainty evidence that there is no statistically significant difference in the rate of adverse events when nitrates are administered to RVMI compared with other cardiac region MIs.
- Hypotension is the primary adverse event reported. Nitrates have a serum half-life of 1 to 4 minutes and therefore hypotension is likely to be transient in nature
- Transient hypotension is not really clinically meaningful, especially since it can be treated with cessation of nitrates and fluid challenges
Author Conclusion: “This review suggests that the AHA and ESC contraindications are not supported by evidence. Key limitations include all studies having concomitant inferior and RVMI, not evaluating beneficial effects and very low certainty of evidence. As adverse events are transient and easily managed, nitrates are a reasonable treatment modality to consider during RVMI on current evidence.”
Clinical Take Home Point: From a clinical perspective, the potential benefit of analgesia and reduced sympathetic stimulation in the setting of RVMI seems to outweigh the potential of transient hypotension with the use of nitrates. Although better studies are needed, this systematic review and meta-analysis argues against a contraindication against nitrates in the setting of RVMI and maybe one of precaution.
References:
- Wilkinson-Stokes M et al. Adverse Events From Nitrate Administration During Right Ventricular Myocardial Infarction: A Systematic Review and Meta-Analysis. Emerg Med J 2023. PMID: 36180168
- Ferguson JJ et al. Significance of Nitroglycerin-Induced Hypotension with Inferior Wall Acute Myocardial Infarction. Am J Cardiol 1989. PMID: 2502902
- Neumar RW et al. Part 1. Executive Summary: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015. PMID: 26472989
- Ibanez B et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation. Eur Heart J 2018. PMID: 28886621
For More Thoughts on This Topic Checkout:
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter/X: @EMSwami)
The post REBEL Cast Ep124: Nitrates in Right Sided MIs? appeared first on REBEL EM - Emergency Medicine Blog.
18 March 2024, 12:00 pm - 4 minutes 13 secondsREBEL Core Cast 119.0 – Sleep Hygiene


REBEL Core Cast 119.0 – Sleep Hygiene
Click here for Direct Download of the Podcast
Employ sleep strategies:
- Anchor sleep: a period of sleep that overlaps each day regardless of your night shift schedule to provide a guidepost for your body clock. Ideally would overlap with when you would normally be asleep if you were not on night shift.
- Split sleep: sleep 3-4 hours immediately after shift then another 3-4 hours immediately before shift
Melatonin timing/dosing:
- Most sleep specialists recommend 1-3 mg 30 minutes before desired onset of sleep
- Align timing to bolster your circadian rhythm, not fight it
Caffeine:
- Limit intake 4-8 hours before bed and no more than 400 mg a day
Diet:
- Choose healthy foods and snacks and consume them in patterns that align with your normal day-night cycle (i.e. eat dinner before your night shift and eat breakfast afterwards)
- Residency/hospital leadership should consider having healthy low-cost/free food options available for residency and staff, particularly on night shifts
Don’t drive sleepy:
- Practice recognizing signs of sleep deprivation (yawning, drifting lanes, falling asleep at signout or at red lights) and do not drive home if present
- Residency/hospital leadership should provide a comfortable place for residents/faculty to sleep and/or provide rideshare options to safely get home when signs of sleep deprivation are recognized
References
Sleep strategies:
- Minors DS, Waterhouse JM. Does ‘anchor sleep’ entrain circadian rhythms? Evidence from constant routine studies. J Physiol. 1983 Dec;345:451-67. doi: 10.1113/jphysiol.1983.sp014988. PMID: 6663508; PMCID: PMC1193807.
- Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Complete or partial circadian re-entrainment improves performance, alertness, and mood during night-shift work. Sleep. 2004 Sep 15;27(6):1077-87. doi: 10.1093/sleep/27.6.1077. PMID: 15532201.
Melatonin
- Farahmand S, et al. Comparison of exogenous melatonin versus placebo on sleep efficiency in emergency medicine residents working night shifts: A randomized trial. World J Emerg Med. 2018;9(4):282-287. doi: 10.5847/wjem.j.1920-8642.2018.04.008. PMID: 30181797; PMCID: PMC6117540.
- Morgenthaler TI et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007 Nov;30(11):1445-59. doi: 10.1093/sleep/30.11.1445. Erratum in: Sleep. 2008 Jul 1;31(7):table of contents. PMID: 18041479; PMCID: PMC2082098.
Caffeine
- Walsh JK, Muehlbach MJ, Schweitzer PK. Hypnotics and caffeine as countermeasures for shift work related sleepiness and sleep disturbance. J Sleep Res. 2009;4;80-83.
- Nehlig A. Interindividual Differences in Caffeine Metabolism and Factors Driving Caffeine Consumption. Pharmacol Rev. 2018;70(2):384-411. doi:10.1124/pr.117.014407
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025. 9th Edition. December 2020. Available at DietaryGuidelines.gov.
- Stephanie Centofanti, Siobhan Banks, Scott Coussens, Darren Gray, Emily Munro, Johnathon Nielsen & Jillian Dorrian (2020) A pilot study investigating the impact of a caffeine-nap on alertness during a simulated night shift, Chronobiology International, 37:9-10, 1469-1473, DOI: 10.1080/07420528.2020.1804922.
Diet
- Lowden A, Holmbäck U, Åkerstedt T, Forslund J, Lennernäs M, Forslund A [2004]. Performance and sleepiness during a 24 h wake in constant conditions are affected by diet. Biol Psychol 65(3):251–263.
- Anderson C, Horne JA [2006]. A high sugar content, low caffeine drink does not alleviate sleepiness but may worsen it. Hum Psychopharmacol 21(5):299–303.
Driving Sleepy
- Barger LK, Cade BE, Ayas NT, et al. Extended work shifts and the risk of motor vehicle crashes among interns. N Engl J Med. 2005;352(2):125-34.
- Green W, Gao X, Li K, et al. The Association of Sleep Hygiene and Drowsiness with Adverse Driving Events in Emergency Medicine Residents. West J Emerg Med. 2020;21(6):219-224. Published 2020 Oct 27. doi:10.5811/westjem.2020.8.47357
- Steele MT, Ma OJ, Watson WA, Thomas HA Jr, Muelleman RL. The occupational risk of motor vehicle collisions for emergency medicine residents. Acad Emerg Med. 1999 Oct;6(10):1050-3. doi: 10.1111/j.1553-2712.1999.tb01191.x. PMID: 10530665.
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter/X: @srrezaie)
The post REBEL Core Cast 119.0 – Sleep Hygiene appeared first on REBEL EM - Emergency Medicine Blog.
6 March 2024, 5:00 pm - 4 minutes 13 secondsREBEL Core Cast 118.0 – IM vs PO NSAIDs

REBEL Core Cast 118.0 – IM vs PO NSAIDs
Click here for Direct Download of the Podcast
Bottom Line Up Top: There is no difference in analgesic efficacy between oral and intramuscular NSAIDs.
Clinical Scenario: A 34-year-old woman presents to the ED with back pain. After your history and physical, you conclude that the patient’s pain is muscular in origin and likely secondary to heavy lifting while moving apartments. You contemplate analgesic options and decide that a NSAID makes sense. Should you give her PO ibuprofen or IM ketorolac?
What Your Gut Says: Give the ketorolac IM. IM ketorolac will provide better pain relief and the patient will be happier with her care since she got an ‘injection’ and, after all, she did come all the way to the hospital.
What The Evidence Says: Unlike with many areas of medicine, there is ample evidence to answer this question and most of that evidence has been around for a couple of decades. Reviewing every study would be tedious and, fortunately, we’ve got a great review article on the topic. One important thing to understand is that the different NSAIDs have widely accepted equianalgesic doses; at the right dose, all NSAIDs (whether it be naproxen, ibuprofen, ketorolac or diclofenac) give equivalent pain relief (Irizarry 2021). This allows us to look at studies with the different NSAIDs and compare them to each other.
A 2007 review of the literature concluded that there was no difference in analgesia between IM ketorolac and PO ibuprofen (Arora 2007). The study included a number of high-quality research studies:
Study
Format
Comparison
Findings
Notes
Retrospective analysis of prospectively collected data.
PO Ibuprofen 800 mg vs IM ketorolac 60 mg
No difference in analgesic effect.
Ibuprofen superior secondary to cost, ease of administration + lack of pain w/ administration.
Double-blind RCT
PO Ibuprofen 800 mg vs IM ketorolac 60 mg
No difference in analgesic effect.
Similar onset of action in mild-moderate pain.
Double-blind RCT
PO Ibuprofen 800 mg vs IM ketorolac 60 mg
No difference in analgesic effect.
Double-blind RCT
PO Ibuprofen 800 mg vs IM ketorolac 60 mg
No difference in analgesic effect.
Surgical Patients
Double-blind RCT
IM diclofenac 75 mg vs PO diclofenac 100 mg
Small difference favoring IM in terms of speed to pain relief
Authors conclude PO superior due to time to prepare injection
The data looks fairly clear in terms of analgesic efficacy but, don’t some patients simply prefer to receive a shot? While this dogmatic claim is often made, the data doesn’t appear to support it.
Schwartz and colleagues performed an ingenious trial (Schwartz 2000)
- Enrolled 64 ED patients with acute pain.
- Treatment arms:
- Oral group: Orange drink (800 mg ibuprofen) + placebo “ibuprofen” pill
- Injection group: Orange drink (800 mg ibuprofen) + placebo “ketorolac” injection
- Essentially, all patients got the same analgesic medication (the orange drink) thinking it was just some juice and an inert study placebo (pill or injection).
- No significant difference in analgesia between the two groups.
Bottom Line: Just give the NSAID by mouth. IM NSAIDs may provide slightly faster time to analgesia but, IM dosing comes with the cost of injection, pain , a longer time to prepare the dose and more intensive nursing resources to administer the medication. As long as the patient’s gut works, oral NSAIDs provide similar analgesic effects to IM dosing and should be the preferred route of administration.
Bonus Pearls:
- IM injection of ketorolac causes significant pain. If the patient can’t take PO, be kind and pop in an IV.
- The ceiling pain relief dose for ketorolac is 15 mg IV (Motov 2017).
Read More
REBEL EM: The Ketorolac Analgesic Ceiling
References
Irizarry E et al. A randomized controlled trial of ibuprofen versus ketorolac versus diclofenac for acute, nonradicular low back pain. Acad Emerg Med 2021; 28(11): 1228-35. PMID: 34133820
Arora S et al. Myth: Parenteral ketorolac provides more effective analgesia than oral ibuprofen. Can J Emerg Med 2007; 9(1): 30-2. PMID: 17391598
Wright JM et al.. NSAID use and efficacy in the emergency department: single doses of oral ibuprofen versus intramuscular ketorolac. Ann Pharmacother 1994;28:309-12. PMID: 8193414
Turturro MA et a. Intramuscular ketorolac versus oral ibuprofen in acute musculoskeletal pain. Ann Emerg Med 1995;26:117-20. PMID: 7618770
Neighbor ML et al. Intramuscular ketorolac vs oral ibuprofen in emergency department patietns with acute pain. Acad Emerg Med; 1998; 5(2): 118-122 .PMID: 9492131
Mixter CG et al. Preemptive pain control in patients having laparoscopic hernia repair: a comparison of ketorolac and ibuprofen. Arch Surg 1998;133:432-7. PMID: 9565125
Qureshi I et al. Intramuscular versus oral diclofenac for acute pain in adults with acute musculoskeletal injuries presenting to the ED setting: a prospective, double-blind, double dummy, randomised controlled trial. 2019; 36: 401-6. PMID: 31217178
Schwartz NA et al. Patient’s perceptions of route of nonsteroidal anti-inflammatory drug administration and its effect on analgesia. Acad Emerg Med 2000; 7: 857-61. PMID: 10958124
Motov S et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med 2017; 70(2): 177-84. PMID: 27993418
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter/X: @srrezaie)
The post REBEL Core Cast 118.0 – IM vs PO NSAIDs appeared first on REBEL EM - Emergency Medicine Blog.
21 February 2024, 5:00 pm - 5 minutes 15 secondsREBEL Core Cast 117.0 – Infections of Pregnancy

 Take Home Points
Take Home Points- Infections are a leading cause of maternal mortality worldwide.
- Prompt recognition is critical in management.
- Most infectious processes will require admission and close observation for improvement or decompensation.
REBEL Core Cast 117.0 – Infections of Pregnancy
Click here for Direct Download of the Podcast
Urinary Tract Infection/Pyelonephritis
Epidemiology:
- Occurs in as many as 15% of pregnant women and between 20-40% of pregnant women with asymptomatic bacteriuria will progress to pyelonephritis (Gorgas 2008)
Management:
- Uncomplicated UTI
- Suggested antibiotics include:
- Nitrofurantoin 100mg PO BID x7 days OR
- Cephalexin 500mg PO BID x7 days
- Suggested antibiotics include:
- Pyelonephritis
- Hospital admission
- Suggested antibiotics include:
- Ceftriaxone 1g IV Q24H OR
- Aztreonam 2g IV Q8H for beta-lactam allergy
Complications:
- Maternal sepsis
- Maternal renal injury
- Congenital abnormalities of the fetus
- Premature rupture of membranes
- Low birth weight
Chorioamnionitis
Definition: Also known as intraamniotic infection. Chorioamnionitis is a bacterial infection of fetal amnion and chorion membranes.
Epidemiology:
- Occurs in 1 to 10% of all pregnancies (Gorgas 2008)
- Incidence increases significantly with preterm labor
Diagnosis:
- Chorioamnionitis is defined as maternal fever >38°C and at least two of the following (Apantaku and Mulik 2007):
- Maternal tachycardia >100 beats/min for five minutes
- Fetal tachycardia >160 beats/min for five minutes
- Purulent or foul-smelling amniotic fluid or vaginal discharge
- Uterine tenderness
- Maternal leukocytosis
Evaluation (Abbrescia 2003):
- CBC
- Blood cultures
- Vaginal fluid for phosphatidylglycerol
- Tests for fetal lung maturity
- Cervical AND vaginal cultures
- Physical Exam
- Avoid digital cervical exam
- Speculum exam should be done with sterile speculum
- Ultrasonography for fetal well being
Management:
- Given concern for neonatal sepsis, patients should be admitted for IV antibiotics, supportive cares, and possible early delivery
- Most commonly an ascending infection from normal vaginal flora, so antibiotics must be chosen to cover polymicrobial infections
- Ex. Ampicillin IV 2g Q6H AND Gentamicin IV 1.5mg/kg Q8H
- In PCN allergic patient substitute vancomycin 1 g IV Q12H for ampicillin
- Can only be considered cured with delivery of infected products of conception
Complications:
- Placental abruption
- Premature birth
- Neonatal sepsis
- Neonatal death
- Cerebral palsy
- Maternal sepsis
- Need for cesarean delivery
- Postpartum hemorrhage
Postpartum Endometritis
Definition: Generalized uterine infection
Epidemiology:
- Sepsis results in 15% of maternal deaths worldwide (Houry 2014)
- More common in surgical than vaginal deliveries
- May co-exist with surgical site infection
Diagnosis:
- Classic triad includes: fever, lower abdominal pain and uterine tenderness, and foul smelling lochia
Management:
- Hospital admission
- Cover for polymicrobial infection, including anaerobes
- Ex. Clindamycin 900 mg IV Q8H AND Gentamicin 5-7 mg/kg IV Q24H
Septic Abortion
Epidemiology:
- The World Health Organization estimates that one in eight pregnancy related deaths worldwide can be directly attributed to unsafe abortion procedures (Gorgas 2008)
Diagnosis:
- Clinical presentation includes fever, abdominal pain and uterine tenderness in setting of recent abortion
- Presentation can vary from mild infection to septic shock
Evaluation:
- Lactate
- Cultures of cervix, blood and urine
- Coagulation panel to screen for DIC
- Abdominal X-ray to evaluate for free air or retained surgical foreign bodies
- Pelvic ultrasound to evaluate for retained products of conception or surgical foreign bodies
Management:
- Hospital admission may be indicated as infection can progress to septic shock, organ failure, DIC and cardiovascular collapse
- Broad-spectrum antibiotics are indicated. Triple antibiotic coverage is recommended. Suggested regimens include:
- Ampicillin AND
- Gentamicin AND
- Clindamycin OR Metronidazole
- Update tetanus vaccination
- Usually requires dilation and curettage to remove any retained products of conception or foreign bodies.
References:
- Abbrescia, K. and B. Sheridan (2003). “Complications of second and third trimester pregnancies.” Emerg Med Clin North Am 21(3): 695-710, vii. PMID: 12962354
- Apantaku, O. and V. Mulik (2007). “Maternal intra-partum fever.” J Obstet Gynaecol 27(1): 12-15. PMID: 17365450
- Desai, S. and S. Henderson. Labor and Delivery and Their Complications. In: Marx, J et al, ed. Rosen’s Emergency Medicine. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:2331-2350.
- Gorgas, D. L. (2008). “Infections related to pregnancy.” Emerg Med Clin North Am 26(2): 345-366, viii. PMID: 18406978
- Houry, D and B. Salhi. Acute Complications of Pregnancy. In: Marx, J et al, ed. Rosen’s Emergency Medicine. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014: 2282-2299.
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter/X: @srrezaie)
The post REBEL Core Cast 117.0 – Infections of Pregnancy appeared first on REBEL EM - Emergency Medicine Blog.
7 February 2024, 5:00 pm - 28 minutes 22 secondsREBEL Cast Ep123: Reduced-Dose Systemic Peripheral Alteplase in Massive PE?
 Background: Massive pulmonary embolism defined as sustained hypotension (SBP <90mmHg) has a high mortality which is why early recognition and thrombolytic therapy is typically recommended (AHA Class IIA; ESC Class IB) [1]. However, full-dose thrombolytic therapy (Alteplase 100mg (IV) is associated with an increase in bleeding [2]. Because the lungs receive 100% of cardiac output, it has been hypothesized that a lower dose of thrombolytic therapy may still be effective with a better safety profile [3][4].
Background: Massive pulmonary embolism defined as sustained hypotension (SBP <90mmHg) has a high mortality which is why early recognition and thrombolytic therapy is typically recommended (AHA Class IIA; ESC Class IB) [1]. However, full-dose thrombolytic therapy (Alteplase 100mg (IV) is associated with an increase in bleeding [2]. Because the lungs receive 100% of cardiac output, it has been hypothesized that a lower dose of thrombolytic therapy may still be effective with a better safety profile [3][4].REBEL Cast Ep123: Reduced-Dose Systemic Peripheral Alteplase in Massive PE?
Click here for Direct Download of the Podcast
Paper: Aykan AC et al. Reduced-Dose Systemic Fibrinolysis in Massive Pulmonary Embolism: A Pilot Study. Clin Exp Emerg Med 2023. PMID: 37188358
Clinical Question: What is the efficacy and safety of low-dose (25mg) prolonged administration (over 6hrs) of alteplase in patients with massive PE?
What They Did:
- Single-center, pilot prospective observational cohort trial in Turkey
- Thrombolysis
- 25mg of alteplase without a bolus was administered over 6 hours by peripheral IV infusion
- If hemodynamic instability persisted despite first dose of thrombolysis, a second 6hr infusion of 25mg alteplase without bolus was administered (No patients in the study required this)
- Did not use concomitant heparin anticoagulation with thrombolysis
- Heparin was administered as a 70U/kg bolus followed by a 1000U/h infusion with a target activated PTT between 1.5 and 2.5x the control started immediately after infusion of thrombolysis completed
- Patients were all converted to warfarin for discharge
- TTE
- All patients underwent TTE before thrombolysis, within an hour after thrombolysis, before discharge (5 to 7d) and a month after thrombolysis
- PASP estimated from tricuspid valve regurgitant jet velocity
- Maximum dimension of RA/LA measured in 4-chamber view
- Diameter and collapsibility of IVC noted
- Pulmonary HTN defined as PASP >40mmHg
- RV enlargement defined as RV/LV ratio >0.9
- Tricuspid Annular Plane Systolic Excursion (TAPSE) also recorded
- Tissue Doppler Derived Tricuspid Annular Systolic Velocity recorded
- Tei-Myocardial Performance Index (MPI/Tei) recorded
- CT
- All patients underwent 64 slice CTPA for definitive diagnosis of PE at admission
- Additional CTPA 24 hours after completion of thrombolysis if eGFR >60mL/min/1.73m2
- Criteria for Thrombolytic Success Included:
- Doppler documentation of resolution of increased PASP (<40mmHg)
- Decreased RV diameter (at least 25% decrease of RV/LV diameter)
- Restoration of RV function (TAPSE>16mm)
- Systolic Wave Prime (S’) >10.0cm/s
- Tissue Doppler Derived RV MPI > 0.55
- Clinical improvement of symptoms and restoration of stable hemodynamic status immediately after thrombolysis
- Complete success = Clinical improvement of symptoms and restoration of a stable hemodynamic status along with at least 3 other criteria without resultant death and nonfatal major complications
Outcomes:
- Primary:
- In-hospital mortality
- Major complications
- Ischemic stroke, ICH, embolism (coronary or peripheral), bleeding requiring transfusion
- Pulmonary HTN while in hospital
- RV dysfunction while in hospital
- Secondary:
- 6 month mortality
- Development of pulmonary hypertension at 6 months
- RV dysfunction at 6 months
Inclusion:
- Adult patients (≥18 years of age)
- Confirmed massive PE
- Massive PE Definition
- Acute PE with sustained hypotension (SBP <90mmHg for at least 15 minutes or requiring inotropic support, not due to a cause other than PE, such as arrhythmia, hypovolemia, sepsis, or LV dysfunction)
- Pulselessness
- Persistent profound bradycardia (HR<40BPM with signs or symptoms of shock)
- Massive PE Definition
Exclusion:
- Prior ICH
- Known structural intracranial cerebrovascular disease (i.e. AV malformation)
- Known malignant intracranial neoplasm
- Ischemic stroke within 3 months
- Suspected aortic dissection
- Active bleeding or bleeding diathesis
- Recent surgery encroaching on the spinal canal or brain
- Recent significant closed-head or facial trauma with radiographic evidence of bony fracture or brain injury
Results:
- 37 consecutive patients with massive PE were enrolled
- Blood pressure recovered within a few hours after initiation of thrombolysis in all cases
- Mortality
- Primary Efficacy Outcome: 1 in-hospital death (3.1% in the paper but 2.7% in table 2) on day 6 of hospitalization due to malignant ventricular arrhythmia (Pt had ischemic dilated cardiomyopathy with LVEF of 20% and acute on chronic renal failure)
- 2 additional deaths within 6 months (Both pts had malignancy)
- Not implicitly stated whether these deaths are attributed to PE, however it doesn’t sound like they are based on the patient descriptions
- TTE Results from Admission to Post Thrombolysis:
- Primary Efficacy Outcome: Mean PASP ≈57mmHg to 34mmHg (p<0.001)
- Mean PASP continued to decrease prior to discharge ≈34mmHg to ≈30mmHg (p<0.001)
- Mean PASP preserved at 6 month follow up ≈29mmHg
- No patients with pulmonary hypertension at 6 months
- Primary Efficacy Outcome: Mean TAPSE ≈1.43cm to 2.07cm (p<0.001)
- RV/LV Diameter ≈1.37 to ≈0.99 (p<0.001)
- Mean MPI/Tei Index≈ 0.47 to ≈0.55 (p<0.001)
- Systolic Wave Prime (S’) ≈10 to ≈15
- Primary Efficacy Outcome: Mean PASP ≈57mmHg to 34mmHg (p<0.001)
- Follow Up CTPA 24hrs After Thrombolysis
- Out of 37 patients, 18 (56.3%) underwent repeat CTPA 24hrs after thrombolysis
- Out of these 18 pts total lysis of thrombus was observed in 16pts (88.9%) and the remaining 2pts had >75% lysis of thrombus
- Complications Post Thrombolysis:
- Primary Safety Outcome: No major bleeding or stroke observed
- 3 patients with minor bleeding
- 2pts with epistaxis (2 days after thrombolysis)
- 1pt with gingival bleeding (3 days after thrombolysis)
- All bleeding events occurred during heparin infusion and stopped with gentle compression without recurrence
- 6pts (17.6%) had major bleeding and 2pts (5.68%) had minor bleeding due to warfarin
- Out of 37 patients, 18 (56.3%) underwent repeat CTPA 24hrs after thrombolysis
Strengths:
- Consecutive patients enrolled which minimizes selection bias
- All patients followed for 6 months (No loss to follow up)
Limitations:
- Single center, nonrandomized observational trial
- No comparison group receiving standard therapy (i.e. compared to half-dose 50mg or full dose 100mg of alteplase)
- Small sample size with few complications makes this an underpowered study to make any firm conclusions about bleeding risks
- Lots of missing methodology
- Unclear what time period patients were recruited
- How strict were authors in consecutive recruitment (? selection bias)
- Unclear therapies prior to lytics (i.e. Pressors, heparin, etc)
- Who performed echos and unclear degree of consistency (Echo has some subjectivity to it)
Discussion:
- PE is a Spectrum of Disease
- Massive PE is also a spectrum of disease
- This study doesn’t appear to include critically ill massive PE patients who are peri-arrest
- Dripping alteplase over 6 hours is most likely not going to be the appropriate therapy in the more severe massive PE patients
- Primary Efficacy Outcome
- Multiple primary efficacy outcomes were listed in this study, which can be problematic when you get multiple outcomes of varying statistical significance. In this trial all the primary efficacy outcomes were statistically significant, and the authors clearly defined what they meant by complete success in this trial
- Complications While in Hospital
- No cases of major bleeding or ICH in this study
- The 3 patients who had minor bleeding at 48 to 72hrs were most likely due to the heparin infusion and not associated with thrombolysis
- The cohort is simply too small with not enough complications for the study to be powered correctly for this outcome
- IV Access
- These authors gave alteplase through a peripheral IV which we do in stroke patients, but that is typically done over 1hr not over 6hrs
- If I was going to do this (25mg alteplase over 6hrs) I would want a central venous catheter to avoid the potential of infiltration or if the patient decompensates further and needs central venous access after the fact there is a higher risk of hematoma/bleeding
- Also, I would already have an arterial line in place before starting thrombolysis for continuous hemodynamic monitoring
- Heparin Dosing
- Heparin was administered as a 70U/kg bolus followed by a 1000U/h infusion with a target activated PTT between 1.5 and 2.5x the control started immediately after infusion of thrombolysis completed
- Prior studies have found marked increase in bleeding when lytics and heparin are given together
- After thrombolysis I typically don’t bolus heparin and just start the infusion
- Also I don’t start the heparin infusion immediately after thrombolysis, I wait for the PTT to be <2x the control before starting
- A dose of 0.5 to 4.0mg/hr typically given in EKOS therapy (See Below). This trial gave 25mg over 6hrs (≈4mg/hr)
- ULTIMA Trial [5]:
- 59pts with massive/submassive PE
- Used alteplase at a dose of 1mg/hr x5hrs, then 0.5mg/hr x10hrs
- Max Dose ≈20mg
- No major bleeding
- SEATTLE II Trial [6]:
- 150pts with massive/submassive PE
- Used alteplase at a dose of 1mg/hr x24hrs
- Max Dose ≈25mg
- 1 severe/life-threatening hemorrhage (Groin hematoma requiring vasopressor support)
- No ICH
- OPTALYSE-PE Trial [7]:
- 101pts with submassive PE
- Used alteplase at a dose of 8mg/2hrs (4mg/hr), 8mg/4hrs (2mg/hr), 12mg/6hrs (2mg/hr), and 24mg/6hrs (4mg/hr)
- Max Dose 24mg
- No major bleeding with 8mg/2hrs, 8mg/4hrs, and 12mg/6hrs
- 2 major bleeding episodes occurred in the 24mg/6hr group
- The max dose any patients got was ≈25mg (Max Dose Range ≈20mg to ≈25mg). Major bleeding seemed to occur in the drips that ran for over 15hrs (3 pts out of 310 [≈1%]); But no cases of major bleeding for drips ≤15hrs
- To take this one step further this trial raises the question of whether EKOS even necessary or is it an overly expensive intervention that potentially increases complications without improving outcomes?
- ULTIMA Trial [5]:
- Although this was not a randomized clinical trial and there was no comparator arm we do have two trials on submassive PE with half-dose alteplase (50mg) given over 2hours [8] and half-dose alteplase (50mg) given in Massive PE [3]
- MOPETT Trial [8]
- 121pts with submassive PE (Called “moderate PE” in the study)
- Randomized to half dose thrombolysis vs anticoagulation alone
- For patients weighing ≥50kg a total dose of 50mg given (10mg bolus by IV push followed by 40mg infusion over 2hrs)
- For patients weighing <50kg a total dose of 0.5mg/kg given (10mg bolus by IV push followed by the remainder over 2hrs)
- Primary endpoints consisted of pulmonary HTN and composite of pulmonary HTN and recurrent PE at 28mos
- Pulmonary HTN at 28mos: 16% half dose thrombolysis vs 63% anticoagulation alone
- Composite Pulmonary HTN and Recurrent PE at 28mos: 16% half dose thrombolysis vs 63% anticoagulation alone
- There were 0 cases of bleeding in either arm
- PEAPETT Trial [3]
- 23 patients with PEA cardiac arrest due to confirmed massive PE
- All pts received 50mg of alteplase as an IV push while CPR was ongoing
- ROSC occurred in 2 to 15 min after alteplase administration in all but one patient
- There was no minor or major bleeding despite chest compressions
- What this current trial [9] is really adding to the literature is an even lower dose of thrombolysis (25mg) efficacious?
- We already know from the MOPETT trial [8] and PEAPETT trial [3] that half-dose alteplase (50mg) had zero cases of bleeding so this current trial just tells us what we already know. 25mg of alteplase has less risk of bleeding than 50mg of alteplase
- Additionally, if 25mg can treat massive PEs [9], this could also be extrapolated to less severe high-risk submassive PEs (Although I would still love to see a head-to-head trial of 25mg vs 50mg)
- MOPETT Trial [8]
Author Conclusion: “Results of this pilot study suggest that low-dose prolonged infusion of tPA is an effective and safe therapy in patients with massive PE. This protocol was also effective in decreasing PASP and restoration of RV function.”
Clinical Take Home Point: Low dose (25mg) alteplase given as a prolonged infusion (over 6hrs) is a promising effective and safe therapy in patients with massive PE and provides an alternative to full dose (100mg) and half-dose (50mg) alteplase. Larger RCTs comparing doses of alteplase are warranted to confirm these findings.
References:
- Jaff MR et al. Management of Massive and Submassive Pulmonary Embolism, Iliofemoral Deep Vein Thrombosis, and Chronic Thromboembolic Pulmonary Hypertension: A Scientific Statement from the American Heart Association. Circ 2011. PMID: 21422387
- Wan S et al. Thrombolysis Compared with Heparin for the Initial Treatment of Pulmonary Embolism: A Meta-Analysis of the Randomized Controlled Trials. Circ 2004. PMID: 15262836
- Sharifi M et al. Pulseless Electrical Activity in Pulmonary Embolism Treated with Thrombolysis (from the “PEAPETT” Study). AJEM 2016. PMID: 27422214
- Wang C et al. Efficacy and Safety of Low Dose Recombinant Tissue-Type Plasminogen Activator for the Treatment of Acute Pulmonary Thromboemolism: A Randomized, Multicenter Controlled Trial. CHEST 2010. PMID: 19741062
- Kucher N et al. Randomized, Controlled Trial of Ultrasound-Assisted Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism. Circ 2014. PMID: 24226805
- Piazza G et al. A prospective, Single-Arm Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACCC Cardiovasc Interv 2015. PMID: 26315743
- Tapson VF et al. A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Reisk Pulmonary Embolism: The OPTALYSE PE Trial. JACC Cardiovasc Interv 2018. PMID: 30025734
- Sharifi M et al. Moderate Pulmonary Embolism Treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013. PMID: 23102885
- Aykan AC et al. Reduced-Dose Systemic Fibrinolysis in Massive Pulmonary Embolism: A Pilot Study. Clin Exp Emerg Med 2023. PMID: 37188358
For More Thoughts on This Topic Checkout:
Post Peer Reviewed By: Anand Swaminathan, MD (X: @EMSwami)
The post REBEL Cast Ep123: Reduced-Dose Systemic Peripheral Alteplase in Massive PE? appeared first on REBEL EM - Emergency Medicine Blog.
29 January 2024, 1:00 pm - 5 minutes 32 secondsREBEL Core Cast 116.0 – Achilles Tendon Rupture

 Take Home Points
Take Home Points- Achilles tendon rupture is a clinical diagnosis. The Thompson Test should be applied in all suspected cases.
- Remember to brace or splint a rupture, even if suspected, in the resting equinus position for optimal healing and prevention of further injury.
- Schedule follow up with orthopedics within 1 week for discussion of operative management vs early rehab protocols.
REBEL Core Cast 116.0 – Achilles Tendon Rupture
Click here for Direct Download of the Podcast
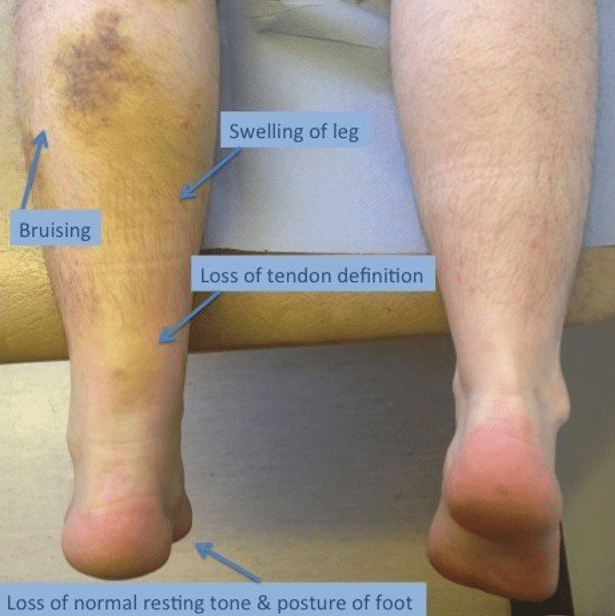 Achilles Tendon Rupture Exam (www.lfaclinic.co.uk)
Achilles Tendon Rupture Exam (www.lfaclinic.co.uk)
Physical Exam
- May have palpable gap or deformity in region of tendon.
- Weakness with plantar flexion.
- Increased resting ankle dorsiflexion on affected side in prone position with knees bent .
- Usually in absence of bony tenderness unless accompanied by other injury
- Thompson Test (video)
- Place the patient in the prone position, with feet hanging over the end of a stretcher or table. If patient is not able to lay down/there are no stretchers, the patient can kneel on a stool or chair
- Squeeze the calf of the normal limb. You will notice the squeeze will cause the ankle to plantarflex appropriately
- Squeeze the calf of the limb with the suspected Achilles tendon rupture. You will notice the squeeze will cause no motion if there is a full rupture/tear, and diminished motion if there is a partial tear
- Performance Characteristics (Garras 2012)
Sensitivity
Specificity
(+) LR
(-) LR
96-100%
93-100%
13.7
0.04
Imaging
- X-Rays
- Used to rule out other or concurrent pathology
- May show soft tissue swelling and destruction of pre-Achilles fat pad (Kager’s Fat Pad)
- Findings are non-specific as tear of tendon unable to be visualized
- Ultrasound
- Ultrasound is helpful if obvious findings present and to distinguish between partial vs complete tears, however only around 50% sensitive for detecting only partial tears (Kayser 2005)
- MRI
- Gold-standard imaging modality
- Rarely, if ever, necessary in the ED
- Used for equivocal physical exam/alternate imaging findings or for assessing the severity of the tear for possible operative management
- Findings
- A full-thickness tear often shows a tendinous gap filled with edema or blood
- Complete rupture shows retraction of tendon ends
ED Management
-
- Provide analgesia
- Tendon stabilization in an optimal healing position
- Functional bracing/splinting in resting equinus/talipus equinus
- AO splint/brace in 20 degrees of plantar flexion for 4-6 weeks (may use tall CAM boot with 20 degrees wedge inserts)
- All patients should be non-weightbearing
- Any weight-bearing can convert a partial tear to a complete tear
- Maintain non-weightbearing status until see orthopedics (within 1 week)
- After evaluation by orthopedics, early weight-bearing and early ROM exercises yield better outcomes (can be as early as 2 weeks)
- Referral to rehab warranted to improve plantar flexion and decrease risk of re-rupture
- ED Ortho consultation: patients with open wounds in the area of trauma, or with concomitant fractures
- Operative Management is usually reserved for acute ruptures (approximately <6 weeks) of full thickness with large tendon gaps, failed conservative treatment of partial thickness tears, or high performance athletes
- These cases will be determined during follow up with orthopedics and may warrant outpatient MRI to assess severity of tear
Prognosis
-
- For conservative management, there is no significant difference in plantar flexion strength (Willits, 2010)
- Some increased risk of re-rupture compared to operative management, although review of evidence shows that this may not be significant if patients used structured, accelerated rehab protocol.
- Protocol includes initially non-weightbearing cast with the foot in equinus position as described above, then transitioned to a pneumatic walker with elevated heels (elevation gradually reduced biweekly), and physical therapy to improve gait, strength, and mobility. (Wallace 2011)
- If addressed early and appropriately, most patients have good self-reported long-term outcomes regardless of the treatment modality
Links
Orthobullets: Achilles Tendon Rupture
Resources:
- Sheth U et al. The epidemiology and trends in management of acute Achilles tendon ruptures in Ontario, Canada: a population-based study of 27,607 patients. Bone Joint J. 2017; 99-B(1): 78-86. PMID: 28053261
- Chiodo CP, Wilson MG. Current Concepts Review: Acute Ruptures of the Achilles Tendon. Foot Ankle Int 2006; 27(4): 305-13. PMID: 16624224
- Leppilahti J, Orava S. Total Achilles tendon rupture. A review. Sports Med. 1998; 25(2): 79-100. PMID: 9519398
- Kayser R et al. Partial rupture of the proximal Achilles tendon: a differential diagnostic problem in ultrasound imaging. Br J Sports Med. 2005; 39(11): 838-42. PMID: 16244194
- Margetic P et al. Comparison of ultrasonographic and intraoperative findings in Achilles tendon rupture. Coll Antropol. 2007; 31:279-284. PMID: 17598414
- Garras DN et al. MRI is Unnecessary for Diagnosing Acute Achilles Tendon Ruptures: Clinical Diagnostic Criteria. Clin Orthop Relat Res 2012; 470(8): 2268-2273. PMID: 22538958
- Willits K et al. Operative versus nonoperative treatment of acute Achilles tendon ruptures: a multicenter randomized trial using accelerated functional rehabilitation .J Bone Joint Surg Am. 2010; 92(17): 2767-75. PMID: 21037028
- Wallace RG et al. The non-operative functional management of patients with a rupture of the tendo Achillis leads to low rates of re-rupture. J Bone Joint Surg Br 2011; 93(10):1362-6. PMID: 21969435
- Erickson BJ. Is Operative Treatment of Achilles Tendon Ruptures Superior to Nonoperative Treatment? Orthop J Sports Med. 2015; 3(4): PMID: 26665055
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter/X: @srrezaie)
The post REBEL Core Cast 116.0 – Achilles Tendon Rupture appeared first on REBEL EM - Emergency Medicine Blog.
24 January 2024, 4:00 pm - 27 minutes 33 secondsREBEL Core Cast 115.0 – Cardiogenic Shock
 Take Home Points: Know clinical (cold extremities, oliguria, confusion, dizziness, narrow pulse pressure) and laboratory markers (metabolic acidosis, elevated creatinine, lactic acidosis) of hypoperfusion. An elevated lactate is a danger sign and requires explanation. Norepinephrine is a great first line vasopressor in Cardiogenic shock. Dobutamine is useful for inotropic support in Cardiogenic shock. Use POCUS ... Read more
Take Home Points: Know clinical (cold extremities, oliguria, confusion, dizziness, narrow pulse pressure) and laboratory markers (metabolic acidosis, elevated creatinine, lactic acidosis) of hypoperfusion. An elevated lactate is a danger sign and requires explanation. Norepinephrine is a great first line vasopressor in Cardiogenic shock. Dobutamine is useful for inotropic support in Cardiogenic shock. Use POCUS ... Read moreThe post REBEL Core Cast 115.0 – Cardiogenic Shock appeared first on REBEL EM - Emergency Medicine Blog.
27 December 2023, 6:00 pm - More Episodes? Get the App
- https://rebelem.com/
- en-US
Your feedback is valuable to us. Should you encounter any bugs, glitches, lack of functionality or other problems, please email us on [email protected] or join Moon.FM Telegram Group where you can talk directly to the dev team who are happy to answer any queries.
 EM Clerkship
EM Clerkship
 Emergency Medical Minute
Emergency Medical Minute
 The Internet Book of Critical Care Podcast
The Internet Book of Critical Care Podcast
 Critical Care Scenarios
Critical Care Scenarios
 EMCrit FOAM Feed
EMCrit FOAM Feed
 Emergency Medicine Cases
Emergency Medicine Cases